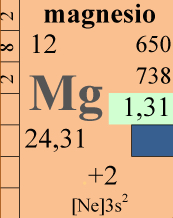
Magnesium is the third element for concentration in our reef tanks and in natural salt water (1200-1500 mg/l) but often we don’t care much about it. Let’s try to analyze its role, starting from who uses it and how.
Reading studies from important universities we know that stony corals and in particular small polyped stony corals (sps) have only 6% magnesium in their skeleton and a further 2% is involved in cellular processes, furthermore this 2% doesn’t affect the total count, since it is continuously recycled without been fixed in the skeleton.
The greater consumers of magnesium are coralline algae that often cover quite big surfaces in reef tanks, from laboratory and in-loco trials we know that coralline algae have a composition of magnesium that varies between 10-15 % in weigh. However whe must keep in mind that those algae have a negligible weight, so their actual consumption is markedly reduced.
Considering those datas it is clear that magnesium is consumed at infinitely lower rates than calcium or carbonates.
Therefore why is magnesium so important for the health and growth of our reefs? Magnesium has the intrinsic ability to bind carbonates with a quite low affinity, such that the link is reversible also at moderate alkaline pH. Magnesium binds, or better sequesters, virtually all the free carbonates creating an unstable compound, in this way only 20% of carbonates is free for a binding with calcium. Here is the reason for what calcium carbonate has supersaturated concentration but doesn’t precipitate as we would expect.
The precipitation doesn’t take place because the mayority part of carbonates are linked to megnesium and it is not available for the link with calcium. Hand by hand the corals adsorb carbonates from the free pool, new carbonates become available thanks to the dissociation from magnesium. The dissociation take place because there is a dynamic equilibrium between free and magnesium-linked carbonates.
If the magnesium concentration falls below 800 mg/l the rate of free carbonates arises very fast and they bind calcium, this condition induce a fast precipitation of calcium carbonate.
Carbonates have a better affinity for calcium than magnesium, however they bind magnesium because of the higher concentration, indeed magnesium is 3 times more concentrated than calcium. It is clear that the stability of the triad (Ca, KH, Mg) strongly depends on the magnesium concentration.
We watched that magnesium has a key role in a reef life, so it is necessary to maintain it at about 1200/1500 mg/l. Most aquarists use magnesium chloride based liquid or powder buffers in order to keep it in the correct range, however, since the consumption is quite limited, a good calcium reactor with natural coral skeletons, can supply the slight request. In case of an anomalous higher consumption, we can just add some magnesium carbonate in our calcium reactor’s chamber, or better set up a post-reactor that uses the residual acidity to dissolve magnesium carbonate (it is enough a less acid pH).